Spotlight on a Researcher – Dr. Dmitriy Minond

 Dmitriy Minond, Ph.D. is an Associate Professor in the College of Pharmacy and a scientist at the Rumbaugh-Goodwin Institute of Cancer Research (RGI) at Nova Southeastern University (NSU). Dr. Minond received his undergraduate degree in Biology and Biochemistry from Odessa State University in Ukraine and Ph.D. in Chemistry and Biochemistry from Florida Atlantic University (Boca Raton, FL). Following his PhD, Dr. Minond pursued postdoctoral research at The Scripps Research Institute in Jupiter, FL. Before joining NSU in 2017 he was an Associate Professor at Harrison School of Pharmacy at Auburn University (2015-2017).
Dmitriy Minond, Ph.D. is an Associate Professor in the College of Pharmacy and a scientist at the Rumbaugh-Goodwin Institute of Cancer Research (RGI) at Nova Southeastern University (NSU). Dr. Minond received his undergraduate degree in Biology and Biochemistry from Odessa State University in Ukraine and Ph.D. in Chemistry and Biochemistry from Florida Atlantic University (Boca Raton, FL). Following his PhD, Dr. Minond pursued postdoctoral research at The Scripps Research Institute in Jupiter, FL. Before joining NSU in 2017 he was an Associate Professor at Harrison School of Pharmacy at Auburn University (2015-2017).
Dr. Minond’s research interests include drug discovery for various diseases. His research has resulted in more than 50 publications in peer-reviewed journals, book chapters and intellectual property.
One of the diseases for which Dr. Minond’s lab is currently conducting research is melanoma. The American Cancer Society estimates that in 2022 about 99,780 new cases of melanoma will be diagnosed, and this skin cancer will cause 7,650 deaths. If detected and treated at an early stage, melanoma cases are mostly curable. When treated before metastasis the 5-year survival rate is 98%, but in cases that remain untreated until metastasis the 5-year survival rate is only 10-15%. Although there are multiple approved therapies, melanoma cancer cells are known to acquire resistance to most of them. Several currently used melanoma treatments also result in toxicity for healthy cells. Moreover, the overall survival of patients suffering from late-stage metastatic melanoma is only about 3 years. Hence, Dr. Minond has been working on developing a more effective therapy against this cancer. He is developing an innovative approach for treating melanoma through targeted inhibition of specific cellular proteins.
The findings of Dr. Minond’s research, regarding small molecules with the potential to be used as a therapy against melanoma, were published in a peer-reviewed article in the journal Cellular Physiology and Biochemistry in 2019. As reported in this article, this novel therapeutic strategy implements a combination of a pharmaceutically-acceptable carrier and a therapeutically active compound that specifically targets certain cellular proteins. The proposed mechanism utilizes anti-melanoma therapeutic compounds that induces basal autophagy and perturbs mitochondrial potential resulting in death of melanoma cells with mutations in BRAF and NRAS genes. Consequently, this mechanism has the potential to be an efficient and specific treatment of metastatic melanoma that has a mutation in BRAF and/or NRAF genetic pathways. The technology presented here represents the first-time proteins such as lamin A/C, DDX1, hnRNP H1/H2 and hnRNP A2/B1 have been used as drug targets for an anti-melanoma targeted therapies such as this one discovered by Dr. Minond. This work holds immense potential to demonstrate therapeutic efficiency against malignant cells as well as decreased deleterious effects on normal cells.
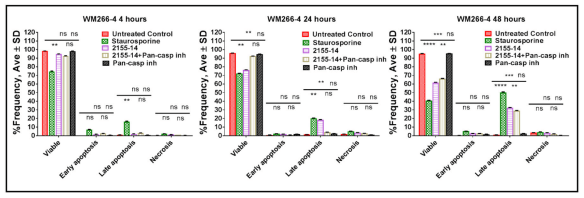
Annexin V assay confirms late apoptosis as one of the mechanisms of 2155–14-induced cell death.
In addition to his research aimed at developing possible therapies for melanoma, Dr. Minond’s lab at RGI has also been focused on developing a novel strategy for treating Rheumatoid arthritis (RA). In the US alone about 1.5 million people are currently living with RA and 71 out of every 100,000 people will be diagnosed with this autoimmune disorder. Although currently there are multiple treatment options for alleviating symptoms, there is no approved cure for RA. Mode of action of currently approved Disease Modifying Anti-Rheumatic Drugs (DMARD) include Janus Kinase (JAK) inhibition, TNF inhibition, T-cell co-stimulation blockade, IL-6 receptor inhibition, B cell depletion, and IL-1 inhibition. Some current medications have demonstrated efficacy as per the American College of Rheumatology 70% (ACR70) improvement criteria. However, more than 50% of RA patients do not respond to any existing treatments. Therefore, there is an unmet need to develop therapies with better efficacy and improved response rate over currently existing options.
Considering this necessity Dr. Minond focused on developing a RA therapeutic that can inhibit a novel target involved in this disease and produce better outcomes both for responders and non-responders of existing therapies. This effort resulted in screening and identification of a specific inhibitor of A Disintegrin And Metalloprotease 10 (ADAM10). This target, ADAM10, is a cell surface enzyme that sheds various cell surface proteins which play important roles in the progression of cancer, inflammatory diseases, and immune response. As both inflammation and immune response are involved in RA, it makes ADAM10 a suitable target for the treatment of this ailment. Findings of Dr. Minond’s research on this novel ADAM10 inhibitor were published in the Scientific Reports journal.
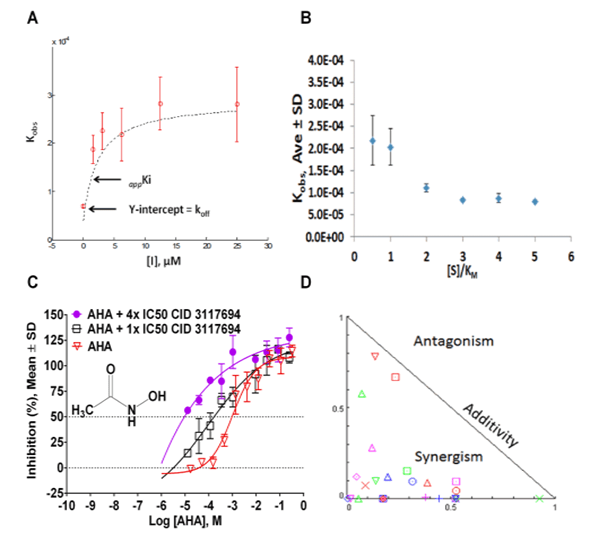
Mechanism of CID 3117694 inhibition of ADAM10
Via High Throughput Screening (HTS), Dr. Minond and his collaborators identified a lead molecule that has the potential to be a selective ADAM10 inhibitor that can address the need for an improved anti-rheumatic drug. This new class of ADAM10 inhibitor acts through a non-Zn binding mechanism and possibly binds to a location that is outside of an active site (exosite). Owing to its binding to a non-Zn binding site, the substrate specificity demonstrated by the lead compound from this class of inhibitors is superior to other currently known ADAM10 inhibitors. It has the potential to be a first in class therapeutic for RA and possibly a new treatment modality for other diseases whose progression involves immune dysfunction and/or inflammation.
The research being conducted in Dr. Minond’s lab has significant potential for developing novel therapeutics for two major disorders. The ADAM10 protease inhibitor can lead to a treatment for RA, while the research with anticancer small molecules has shown potential against melanoma. Considering the therapeutic potential of his research, NSU’s Office of Technology Transfer (OTT) in the Division of Research and Economic Development (DoR) facilitated protection of intellectual property associated with Dr. Minond’s innovations. Currently, OTT has filed patent applications for two novel therapies, one for melanoma and the other for RA, that originated from the research work being done in Dr. Minond’s lab. Currently, both of these technologies are available for licensing through NSU’s OTT so they can be further developed for commercialization.
Dr. Minond’s research at NSU’s RGI will result in further innovation and possible therapies for melanoma, RA, and possibly other diseases in the future. His dedication to the field of drug discovery will enable him to continue conducting research, serving students at NSU, and contributing to the development of novel therapies that will benefit patients.